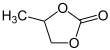
Propylene carbonate
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Methyl-1,3-dioxolan-2-one | |||
| Other names
(RS)-4-Methyl-1,3-dioxolan-2-one
Cyclic propylene carbonate Carbonic acid propylene ester Cyclic 1,2-propylene carbonate Propylene glycol cyclic carbonate 1,2-Propanediol carbonate 4-Methyl-2-oxo-1,3-dioxolane Arconate 5000 Texacar PC | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.003.248 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H6O3 | |||
| Molar mass | 102.089 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.205 g/cm3 | ||
| Melting point | −48.8 °C (−55.8 °F; 224.3 K) | ||
| Boiling point | 242 °C (468 °F; 515 K) | ||
| Very soluble (240 g/L at 20°C) | |||
Refractive index (nD)
|
1.4189 | ||
| Structure | |||
| 4.9 D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Irritant | ||
| GHS labelling:[3] | |||

| |||
| Warning | |||
| H319 | |||
| P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 132 °C (270 °F; 405 K) | ||
| 455 °C (851 °F; 728 K) | |||
| Safety data sheet (SDS) | MSDS by Mallinckrodt Baker | ||
| Related compounds | |||
Related compounds
|
Ethylene carbonate Dimethyl carbonate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Propylene carbonate (often abbreviated PC) is an organic compound with the formula C4H6O3. It is a cyclic carbonate ester derived from propylene glycol.[4] This colorless and odorless liquid is useful as a polar, aprotic solvent.[5] Propylene carbonate is chiral, but is used as the racemic mixture in most contexts.
Preparation
[edit]Although many organic carbonates are produced using phosgene, propylene and ethylene carbonates are exceptions. They are mainly prepared by the carbonation of the epoxides[5] (epoxypropane, or propylene oxide here):
- CH3CHCH2O + CO2 → CH3C2H3O2CO
The process is particularly attractive since the production of these epoxides consumes carbon dioxide. Thus this reaction is a good example of a green process. The corresponding reaction of 1,2-propanediol with phosgene is complex, yielding not only propylene carbonate but also oligomeric products.
Propylene carbonate can also be synthesized from urea and propylene glycol over zinc acetate.[6]
Applications
[edit]As a solvent
[edit]Propylene carbonate is used as a polar, aprotic solvent.[7] It has a high molecular dipole moment (4.9 D), considerably higher than those of acetone (2.91 D) and ethyl acetate (1.78 D).[1] It is possible, for example, to obtain potassium, sodium, and other alkali metals by electrolysis of their chlorides and other salts dissolved in propylene carbonate.[8]
Electrolyte
[edit]Due to its high relative permittivity (dielectric constant) of 64, it is frequently used as a high-permittivity component of electrolytes in lithium batteries, usually together with a low-viscosity solvent (e.g. dimethoxyethane). Its high polarity allows it to create an effective solvation shell around lithium ions, thereby creating a conductive electrolyte. However, it is not used in lithium-ion batteries due to its destructive effect on graphite.[9]
Other
[edit]Propylene carbonate can also be found in some adhesives, paint strippers, and in cosmetics.[10] It is also used as plasticizer. Propylene carbonate is also used as a solvent for removal of CO2 from natural gas and synthesis gas where H2S is not also present. This use was developed by El Paso Natural Gas Company and Fluor Corporation in the 1950s for use at the Terrell County Gas Plant in West Texas, now owned by Occidental Petroleum.[11]
Propylene carbonate product may be converted to other carbonate esters by transesterification as well (see Carbonate ester#Carbonate transesterification).[5]
In electrospray ionization mass spectrometry, propylene carbonate is doped into low surface tension solutions to increase analyte charging.[12]
In Grignard reaction propylene carbonate (or most other carbonate esters) might be used to create tertiary alcohols.[13]
Safety
[edit]Clinical studies indicate that propylene carbonate does not cause skin irritation or sensitization when used in cosmetic preparations, whereas moderate skin irritation is observed when used undiluted. No significant toxic effects were observed in rats fed propylene carbonate, exposed to the vapor, or exposed to the undiluted liquid.[14] In the US, propylene carbonate is not regulated as a volatile organic compound (VOC) because it does not contribute significantly to the formation of smog and because its vapor is not known or suspected to cause cancer or other toxic effects.[15]
See also
[edit]References
[edit]- ^ a b Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1-4398-5511-0.
- ^ Propylene carbonate at Sigma-Aldrich.
- ^ GHS: GESTIS 070730
- ^ WebBook page for propylene carbonate.
- ^ a b c Hans-Josef Buysch. "Carbonic Esters". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_197. ISBN 978-3527306732..
- ^ Synthesis of propylene carbonate from urea http://pubs.acs.org/doi/abs/10.1021/ie049948i
- ^ Dieter Stoye. "Solvents". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_437. ISBN 978-3527306732..
- ^ J. Jorné; C. W. Tobias (1975). "Electrodeposition of the alkali metals from propylene carbonate". J. Appl. Electrochem. 5 (4): 279–290. doi:10.1007/BF00608791. S2CID 93629501.
- ^ Doron Aurbach (1999). Nonaqueous Electrochemistry. CRC Press. ISBN 978-0824773342.
- ^ Propylene carbonate in the Consumer Product Information Database.
- ^ Schendel, R. "Comparison of Fluor Solvent and Selexol Processes" (PDF). Retrieved 4 April 2016.
- ^ Teo C. A., Donald W. A. (May 2014). "Solution additives for supercharging proteins beyond the theoretical maximum proton-transfer limit in electrospray ionization mass spectrometry". Anal. Chem. 86 (9): 4455–62. doi:10.1021/ac500304r. PMID 24712886.
- ^ Payne, Richard; Theodorou, Ignatius E. (September 1972). "Dielectric properties and relaxation in ethylene carbonate and propylene carbonate". The Journal of Physical Chemistry. 76 (20): 2892–2900. doi:10.1021/j100664a019. ISSN 0022-3654.
- ^ "Environmental Profile for Propylene Carbonate". U.S. Environmental Protection Agency. 1998.
- ^ Johnson, William L. "REVISION TO DEFINITION OF VOLATILE ORGANIC COMPOUNDS - EXCLUSION OF PROPYLENE CARBONATE AND DIMETHYL CARBONATE". US Environmental Protection Agency. US EPA. Retrieved 11 July 2015.